

Oncocross
Oncocross is an Artificial Intelligence-based pharmaceutical company specializing in drug discovery/cancer drug development.
View Full Pitch
Highlights
- AI based biotech company specializing in drug development with patented AI algorithm
- Mitigate drug development risk by saving upto 8 years and 95% of the cost
- First AI biotech in Korea to L/O drug candidate to a traditional pharmaceutical company
- Proprietary database set of 100,000+ patients transcriptomic data, 400+ disease signature data, 19+ cohorts of rare disease data, 25,000+ drug signature data, 40 main types of cancer with 79 subtypes
- 16 pipelines and 5 will begin clinical trials within 12-month
- Collaborating with 60+ big pharmas, research institutes, universities, and hospitals
- Raised KRW16.5bn (USD14mn, 140% of targeted amount) in Series B funding this August
Company Overview
Oncocross is an Artificial Intelligence based biotech specializing in drug development.
Oncocross’ service is based on the two AI platform described below:
ONCO AI – is an AI platform to screen optimal indications for drug compound in preclinical or clinical stages and to find the most suitable combination drugs to develop Incrementally Modified Drug (IMD) or orphan drugs, using proprietary human transcriptomic data (RNA expression), disease data, and drug data. Using ONCO AI, we can accelerate drug development process in a shorter time at a lower cost.
ONCOfind AI – is an AI platform to screen and identify the most suitable biomarker for anti-cancer drug compound candidate using proprietary standardized human transcriptomic data of multiple races and various phenotype information of cancer. This platform can also identity the original anatomic site of primary cancer tumor for Carcinoma of Unknown Primary (CUP).
Problem Overview
Solution Overview
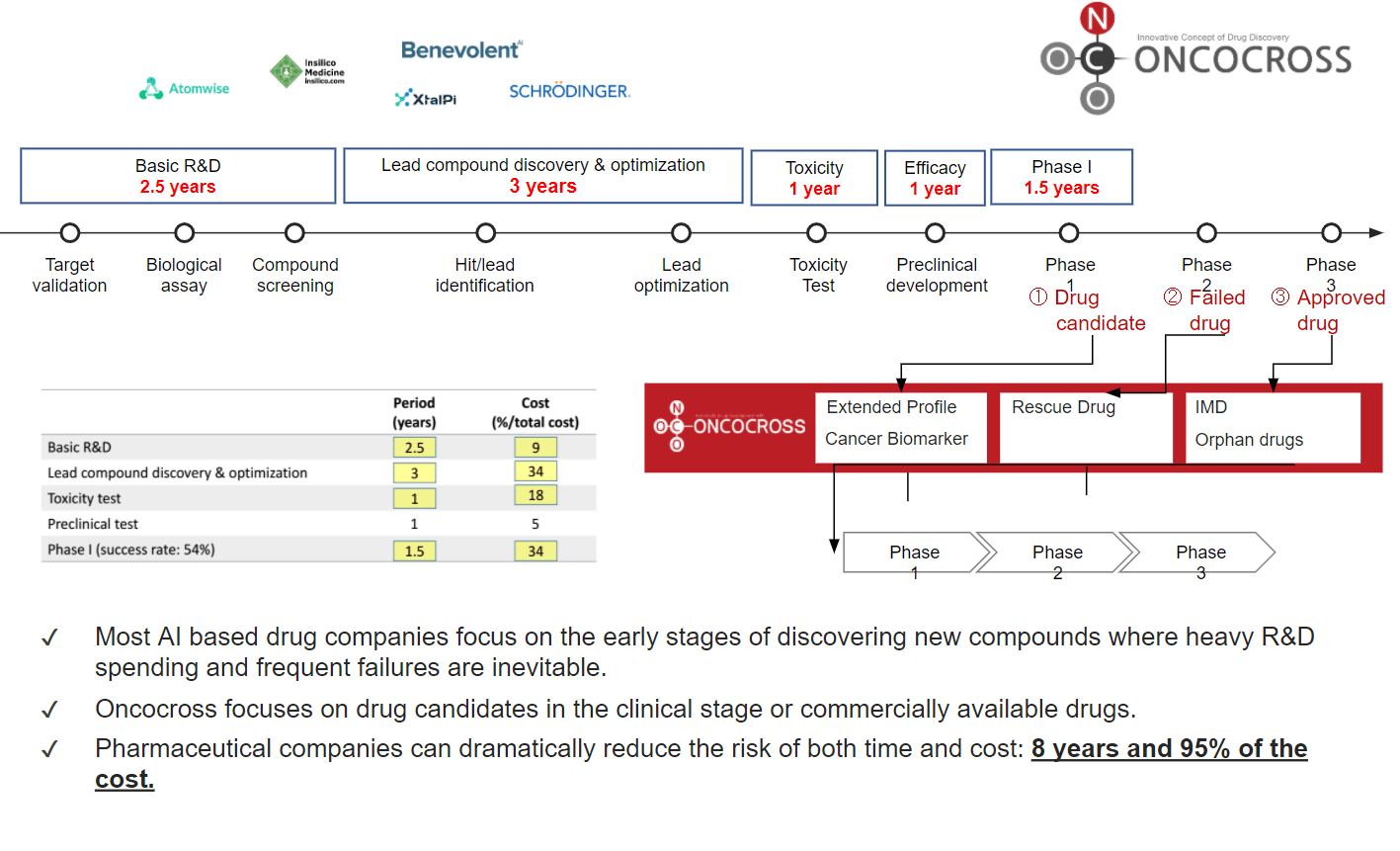
- Extended Profile
- Cancer Biomarker
- Incrementally Modified Drug (IMD)
- Orphan Drug
- Rescue Drug
Go-to-Market Strategy and Business Model
- Revenue model
-
- License-Out
- upfront fee
- milestone fees: enter clinical studies, IND submission, FDA approval
- royalty: when the drug is sold to market
- License-Out
-
- Co-develop and share milestone fee
- Service fee
- find the optimal biomarker for anti-cancer drug compound
- Pricing
-
-
- L/O depends on the type of disease, clinical trial phase
- orphan drug prices are very high
-
- Sales and distribution model
-
-
- business development team: 2 domestic, 2 US, 1 China
-
- Customer pipeline list
-
-
- Novo Nordisk, Boehringer Ingelheim, ST Pharm, Korea Pharma, Daewoong Pharmaceutical
-
Competitive Landscape
Market size:
[ninja_tables id=”7265″]
Resue Drug
- on average approximately 4,500 drugs and medical devices are pulled from the market each year in the U.S.
- only 14% of all drugs in clinical trials eventually win FDA approval according to new MIT study.
- assuming average USD2.6bn spent for one approval, USD2.23bn are sunk cost.
Competition:

- Oncocross AI platform is powered by the proprietary data set – homo sapiens’ transcriptomic data, drug data, disease data and patented AI algorithm ‘ReDRUG’ to screen and match drug and disease.
Founders' Bio

Yirang Kim
CEO
- Ph.D., Medical Science, KAIST - Medical Oncologist (Hematology), - Asan Hospital, Internal Medicine, Department of Oncology, -Visiting Scholar, Wellman Center for Photomedicine, Harvard Medical School, - Visiting Scholar, MIPS (Molecular Imaging Program at Stanford), School of Medicine, Stanford University"

Jinwoo Choi
CTO
- Ph.D. Pharmacology, Seoul National University - Professor, College or Pharmacy, Kyung Hee University
- About Oncocross
- Website : oncocross.com
- Location : Seoul, South Korea
- Founded year : 2015
- Company size : 18 people
- Total funding amount: $19.37 Mil
- Markets: Artificial intelligenceHealthcare
